TALVEY® is administered via subcutaneous injection by a healthcare provider Q2W or QW following the step-up dosing schedule1

Following step-up dosing, ongoing biweekly dosing begins.
Maintain a minimum of 12 days between Q2W doses.
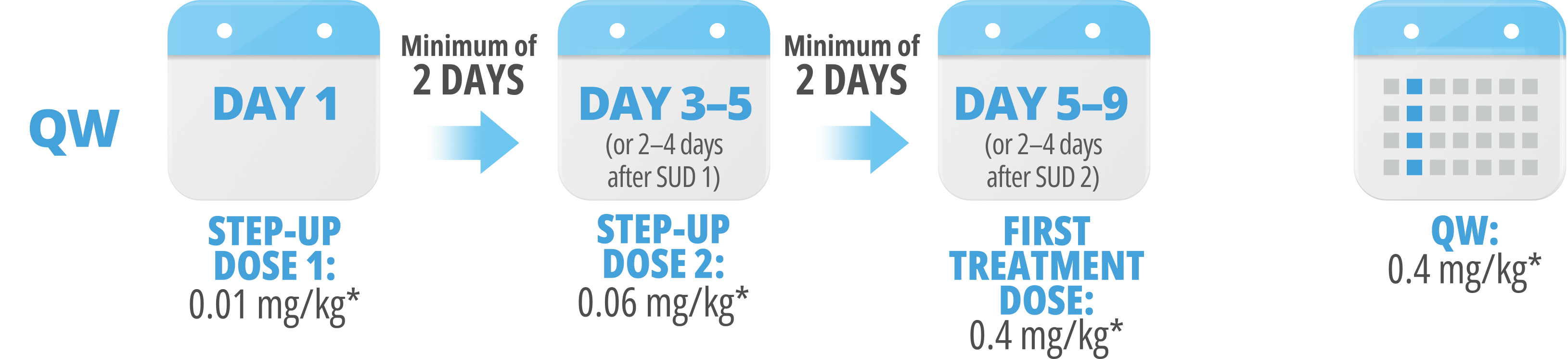
Following step-up dosing, ongoing biweekly dosing begins.
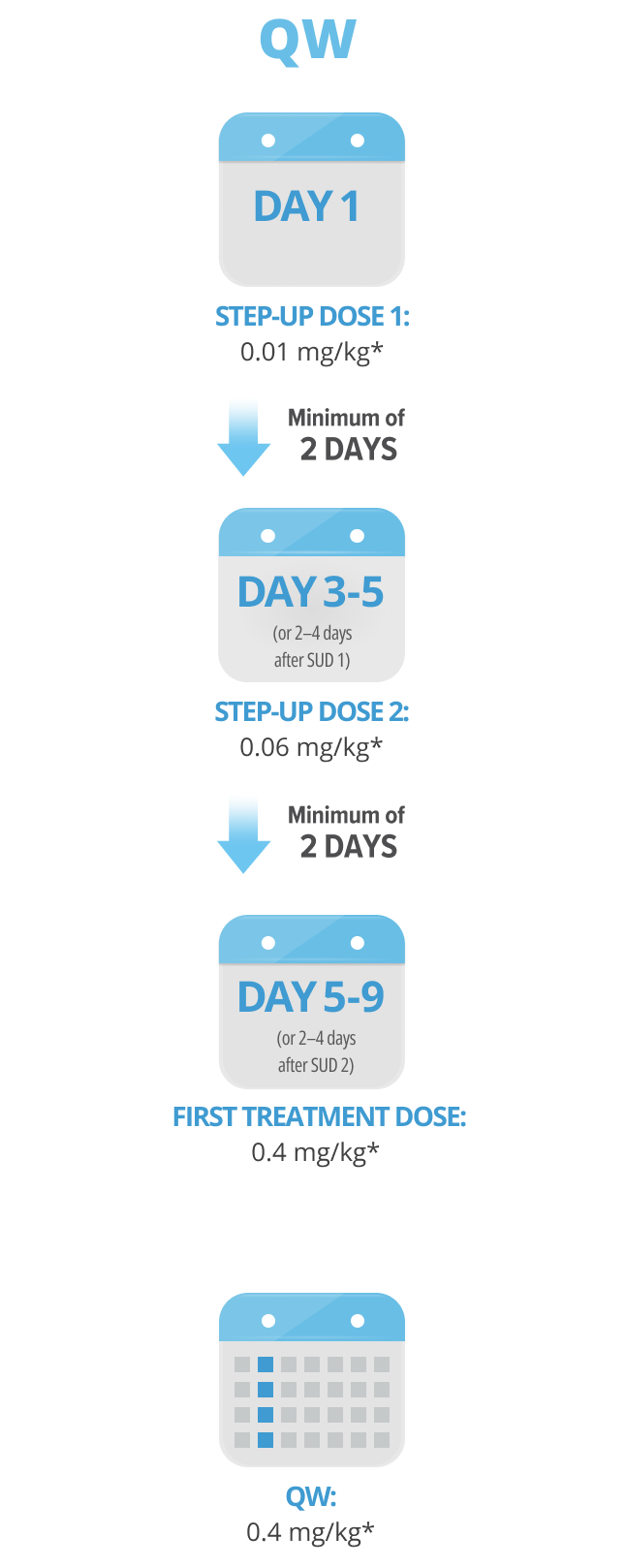
Maintain a minimum of 6 days between QW doses.
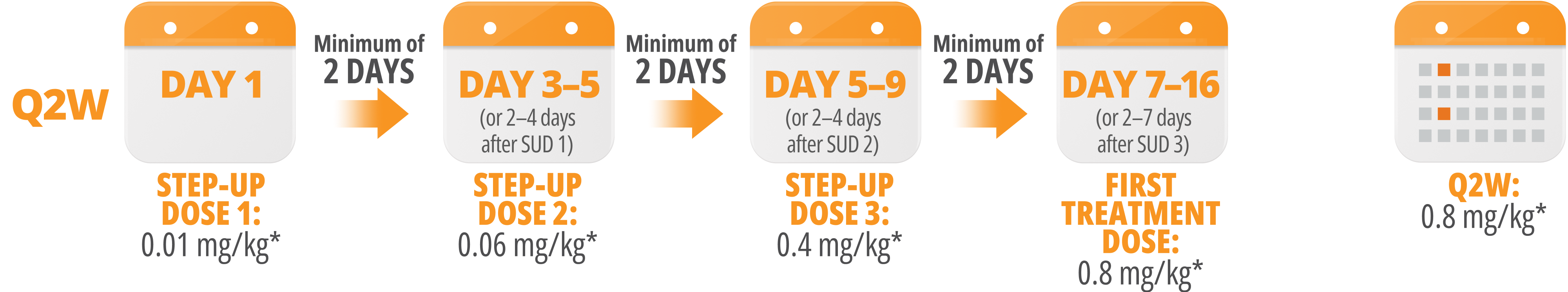
Following step-up dosing, ongoing biweekly dosing begins. Maintain a minimum of 12 days between Q2W doses.
Following step-up dosing, ongoing biweekly dosing begins. Maintain a minimum of 6 days between QW doses.
TALVEY® is given until disease progression or unacceptable toxicity.
You make the move with step-up dosing flexibility:
Step-up doses may be administered between 2 to 4 days after the previous dose and may be given up to 7 days after the previous dose to allow for resolution of adverse reactions. If time is not needed to resolve an adverse reaction, the full step-up dosing schedule can be completed in 7 days for Q2W and 5 days for QW.
Dosing considerations1
Initiate TALVEY® treatment with step-up dosing to reduce the risk of CRS.
Dose delays may be required to manage toxicities.
Preparation and administration considerations1,2
Due to the risk of CRS and neurologic toxicity, including ICANS, patients should be hospitalized for 48 hours after administration of all doses within the TALVEY® step-up dosing schedule.
Rapid subcutaneous injection: TALVEY® does not require the wait time associated with infusions.1,2†
Ready-to-use solution without need for dilution.
Subcutaneous injection into abdomen (preferred) or thigh.
Use aseptic technique to prepare and administer TALVEY®.
Do not combine TALVEY® vials of different concentrations to achieve treatment dose.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Check that the TALVEY® solution for injection is colorless to light yellow. Do not use if the solution is discolored, cloudy, or if foreign particles are present.
Personalized weight-based dosing1
Please refer to Tables 9-12 in the full Prescribing Information to determine total dose, injection volume, and number of vials required.
Pretreatment medications1
1 to 3 hours before each step-up dose
Administer the following pretreatment medications before each dose in the step-up dosing schedule to reduce the risk of CRS
- Corticosteroid (oral or intravenous dexamethasone 16 mg or equivalent)
- Antihistamines (oral or intravenous diphenhydramine 50 mg or equivalent)
- Antipyretics (oral or intravenous acetaminophen 650 mg to 1000 mg or equivalent)
Subsequent doses
Administration of pretreatment medications may be required for subsequent doses for patients who repeat doses within the TALVEY® step-up dosing schedule due to dose delays or for patients who experienced CRS.
Dose modifications
View dose modification recommendations for the management of:
Based on actual body weight.
Supplied as ready-to-use solution for injection that does not require dilution prior to administration.
CRS, cytokine release syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome; QW, once weekly; Q2W, every 2 weeks; SUD, step-up dosing.
- TALVEY® [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.
- Kim H, Park H, Lee SJ. Effective method for drug injection into subcutaneous tissue. Sci Rep. 2017;7(1):9613.

